The Goddess happily sleeps, kept warm by the blanket of Earth’s atmosphere. (image made using Bing)
I often found that non-specialists can explain complex matters more clearly than specialists. So, here is my take as a non-specialist on the basic elements of Earth’s temperature regulation. Don’t take it as more than a “level zero” explanation, but I thought it could be useful for those of us who don’t have the time to delve into the obscure details of atmospheric physics, one of the most complex fields of science you may face. I took a somewhat different approach than usual, which I hope you’ll find useful.
Let’s start with something you are surely familiar with if you are an Earthling: the higher you climb, the cooler the air becomes. It is one of those things everyone knows but few can explain. But it is the key to understanding Earth’s climate. Let me try to explain the story the best I can.
Imagine you are looking at Earth from space. You are familiar with the “blue marble” satellite images showing seas, continents, and clouds. You can see these features at the surface because the atmosphere is mostly transparent to solar light, which happens to be the range of wavelengths where a yellow star, such as the sun, emits the most light. What we see here is light directly reflected into space from Earth.
Now, there is a rule that says, more or less, that what goes in, must go out. Earth receives energy from the Sun and must beam out the same amount. Otherwise, it would be warming forever, and that’s not possible. But the light emission from Earth will be only in part reflected visible light. Because of a very basic thermodynamic principle, Earth will emit the energy it receives in a “degraded” form, that is, at much lower temperatures than the sun (fortunately so, from our viewpoint).
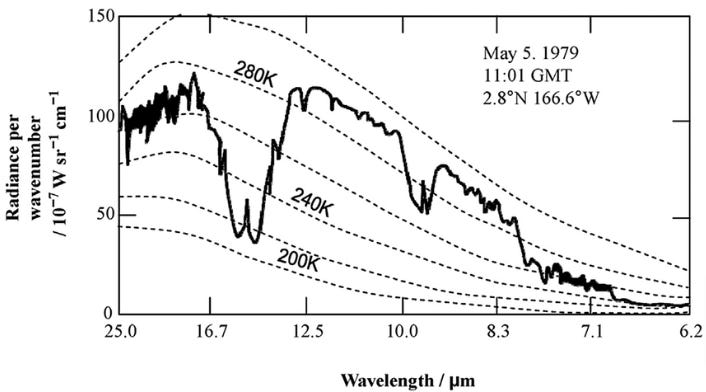
Now, you surely heard the concept of “black body” radiation. It is an abstraction for how a featureless body at a certain temperature would beam heat out. The figure shows what the spectrum should look like if Earth were a black body (dashed lines), together with what it is from actual measurements (continuous line). The scale is in micrometers (μm), millionths of a meter. The visible part of the spectrum is outside the figure, on the right. What we are seeing is the whole band called “infrared”.
Clearly, Earth is not a black body, although it roughly follows the expected spectrum for a temperature of ca 290 K (17 °C), which is close to the average surface temperature measured by thermometers, about 15 °C.
You immediately see that the atmosphere is transparent in some wavelengths but not in others. The zone between 10-12 μm is the “atmospheric window.” what you see there comes directly from the surface and it is a measure of its temperature. It is called the “Land Surface Temperature” (LST) and it is an alternative to using thermometers at ground level that provides similar results. (*)
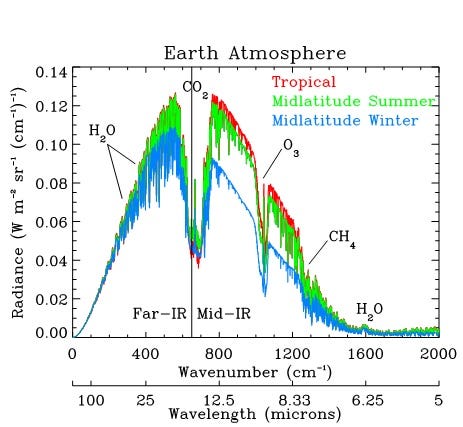
But what are these big “dips” in the spectrum? They are absorption “bands,” part of the spectrum in which light is absorbed by "infrared active” molecules. Take a look at this image, the “radiance” (emitted radiation) measured from space. (source)
As you see, the spectrum is dominated by the big “dip” at ca. 15-16 μm which corresponds to absorption from CO2. It is huge, and people are often surprised because they know that the concentration of CO2 in the atmosphere is only 0.04%. How can such a small amount generate such a big effect?
It can. It is because what counts is not the concentration but the absolute amount. An infrared photon emitted from Earth’s surface doesn’t “see” the oxygen or nitrogen molecules that compose most of the atmosphere. It only sees the infrared-active molecules, CO2, water, and a few more. On the average, it won’t go farther than a few meters before it is absorbed (it is called the “mean free path”). So, its probability of escaping to space is practically zero.
It follows that if you look at the Earth in the wavelengths where CO2 absorbs so much, you would see only a featureless sphere, no traces of the surface features, continents, and the like (**), just the infrared photons emitted by the upper layer of the atmosphere, typically higher than ca. 5 km. There, the atmosphere is rarefied enough that an infrared photon has a decent chance to escape to space.
We can call the CO2 band a “blanket” (if so inclined, you can say, “Gaia’s blanket.”) I think it is better than using the term “greenhouse,” which is not wrong, but it has often been misunderstood.
Of course, a blanket is not the same thing as the atmosphere. A blanket is a uniform layer that slows down the flow of heat from a hot source to a cold sink. The atmosphere, instead, is an incredibly complicated entity that becomes less dense with altitude. It acts as a thermal engine, transforming heat into mechanical energy (it is called “wind”) and also as an air conditioner, moving heat from one height to another by water evaporation and condensation (the result is called “clouds”). And much more. But the term “blanket” emphasizes that CO2 does exactly what a blanket does. It absorbs some of the heat emitted from a hot surface and sends it back to the emitter, Earth’s surface, warming it.
You could define a blanket’s performance by measuring the difference in temperature from the back to the front. The part in contact with the hot surface has to be warmer, of course. Note also that if the system has to be stable, the outer surface of the blanket has to radiate to the environment exactly as much heat as it is being generated from the source. Which means that it has to be at the same temperature the source would be at without the blanket.
We could call “Lapse Rate” the ratio of the difference in temperature between the outside and the inside of the blanket divided by the blanket’s thickness (taken as positive when the top is colder than the bottom). The higher the lapse rate, the more insulating the blanket is. If the lapse rate is zero, the top is at the same temperature as the bottom. Tthe blanket doesn’t insulate anything.
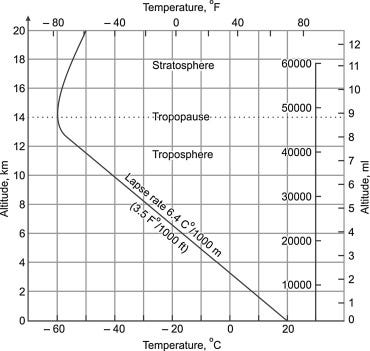
The same considerations hold for the atmosphere. Here is a picture of the lapse rate (from Science Direct):
You see that the atmosphere works as a blanket up to some 12-13 km, the limit of the Troposphere, the region where the air motion is turbulent. Then, the trend is reversed: the stratosphere (laminar motion) is definitely NOT a blanket. It is because the concentration of CO2 per unit volume is so low that it doesn’t significantly stop infrared radiation anymore. The stratosphere warms by a completely different mechanism involving ultraviolet radiation absorption by oxygen and ozone.
So, we have arrived at the answer to why mountains are colder than plains. It is because of the positive lapse rate of the troposphere. The experimental lapse rate is approximately 6.4 C/km, which explains why you need to climb just a few hundred meters to notice a significant cooling. Note also that no mountain is so tall that it emerges into the stratosphere; otherwise, it would become hotter with height!
Now, one last question: what causes global warming?
You have global warming when you add CO2 to the atmosphere (we may neglect other gases as a first approximation). In terms of the “blanket” metaphor, it simply makes the blanket thicker. And you know that if you add a blanket when you feel cold, it will work exactly this way.
Going a little more into the details, when you add CO2 to the atmosphere, it will distribute itself evenly according to how pressure drops with height. It means that there will be more CO2 at those heights where the atmosphere is thin enough to let some infrared radiation escape to space, and emissions will now start from higher heights. But because of the positive lapse rate, it will be colder at those heights and the emitted power will be lower. Hence, the whole big slab of atmosphere and surface will have to warm up a little to compensate.
A bit hard to digest, I know, but don’t worry. Just remember that a thicker blanket makes you warmer! That’s the essence of global warming.
If you could find the time to read all the way to here, congratulations! Now you have a basic level of understanding of what determines Earth’s surface temperature.
I did my best by taking an approach to this matter that’s a little different from the typical ones you find on the Web. I did it mainly because of a personal effort to understand this matter and also to attempt to convey something of how incredibly complex and fascinating atmospheric physics is. For me, every time I return to studying one of its facets, I learn something new. You can try that yourself, it is an incredibly beautiful story. The kind of beauty that can kill, unfortunately for us.
The image below is not taken by a NASA satellite, but, who knows? One day, we might find the right wavelength to see the Goddess Gaia!
___________________________________________________________________
(*) Here is an example of an image taken in the “infrared window” (from ESA). This image is heavily processed to remove clouds and oceans, but it shows what kind of measurements you can make by using different wavelengths.
(**) You would search in vain in the NASA databases for a global picture of Earth’s emissions at 16 micron. It does not exist for two reasons: the first is that it is not interesting to scan the whole globe in a wavelength where you don’t see anything interesting. The second is that the emission comes from a region where temperatures are some -70 C, and it is so weak that sensors have a hard time recording it. The best you can find are images at a wavelength of 13.6 micron, on the “shoulder” of the CO2 band. It is called “ABI-16”, where ABI stands for “Advanced Baseline Image” and “16” indicates the 16th channel of the instrument. It is used to measure CO2 concentrations in different regions. Note that there are plenty of global images around labeled with “IR pictures of Earth” without any indication about which IR range is being measured. For the specialists, it is obvious, but not for us, poor people trying to understand. Here are images of all the 16 ABI channels (link)










Very good – isn’t there a very simple (simplistic?) explanation?
As you move upwards, there are fewer molecules per unit volume. Since it is the molecules that absorb and radiate heat, it follows that the temperature must fall with greater elevation?
Thank you for the blanket-thickness explanation, Ugo.
It is conceptually easy and comfortable.