Oxygen and Carbon in the Atmosphere: the Critical Balance of Life
Gorshkov and Makarieva were right with the idea of the "biotic regulation" of the biosphere.
This post contains a lot of numbers, sorry about that, but atmospheric physics is a complicated story. Even the Goddess Gaia gets confused sometimes. But if you find the time to read it, I think you’ll find the story fascinating. It seems that the balance between oxygen and carbon in the ecosystem is in large part controlled by the powerful mechanism that Gorshkov and Makarieva called “biotic regulation.” Powerful, yes, but not all-powerful, and it can be overwhelmed by an excess of emitted CO₂.
In this post, I am putting together some numbers to understand how the mechanisms of gas exchange in the atmosphere work, and how the system may control the atmospheric composition. It is probably something already known, but likely buried in the pages of some paywalled scientific article. So, I thought to redo the calculations myself, and I think I can confirm, at least qualitatively, that the “biotic regulation” proposed by Gorshkov and Makarieva is real. The ecosphere is not an inert gas container but something that reacts actively to perturbation. It could react so actively that it could wipe us out, but let’s go into the details.
Let me start with something that’s not often discussed. Everybody speaks about CO₂, but what’s happening to O2 in the atmosphere? It is being consumed, no doubt about that. Here are some data from the Mauna Loa Observatory, the same that provides data on CO₂ concentrations (Source)
Oxygen is measured here as 'per meg,' which is a somewhat abstruse unit of measurement used in atmospheric science only. See at the end of this post how it is related to the oxygen concentration in ppmv (parts per million in volume). According to the data, we are losing about 24 per meg units per year, which corresponds to about 5 ppmv of oxygen. The total loss of about 800 per meg of oxygen since 1991 corresponds to a loss of about 165 ppmv.
Before you start worrying, think that the oxygen concentration in the atmosphere in weight is about 23% (21% in volume). That is, 209,500 ppmv. 165 ppmv of oxygen loss is infinitesimal in comparison to the total, about 0.078%. You are not going to choke soon. Incidentally, the fact that oxygen is being consumed is a strong indication that it is the result of burning fossil fuels. Some people say that it is not CO₂ that causes warming, but it is warming that causes CO₂ to be emitted by the hotter ocean. But, if it were the case, oxygen wouldn’t be consumed.
So, let’s go into the details. Burning fossil fuels generates about 36 Gt (Gigatons) of carbon dioxide per year (it may be more, but let’s stick to this value.), or, if you prefer, ca. 10 Gt of carbon. We also know that 1 ppm of CO₂ in the atmosphere corresponds to 2.13 Gt of carbon. So 36 Gt of carbon dioxide correspond to a yearly increase of ca. 6 ppm. But, in reality, we only observe an increment of 2-3 ppmv. Where is the rest of the CO₂ going?
Let’s recap using comparable units: at the same temperature, the number of gas molecules in a certain volume is approximately independent of their composition. (it is the ideal gas law)
CO₂ emissions: ca. 6 ppmv/year
CO₂ observed increase in the atmosphere: ca. 2-3 ppmv/year
Atmospheric Oxygen decrease: ca. 5 ppmv
Now, chemistry is an exact science: one molecule of oxygen creates one molecule of CO2 when it reacts with one atom of carbon. Conversely, photosynthesis creates one molecule of oxygen while one molecule of CO₂ is converted into glucose. So, you would expect that the ppmv of O2 and CO₂ would exchange with each other one for one. The oxygen decrease matches relatively well with the CO₂ emissions, although there is a remaining mismatch of about 1 ppmv/year. More importantly, not all the CO₂ created by burning fuels appears in the atmosphere.
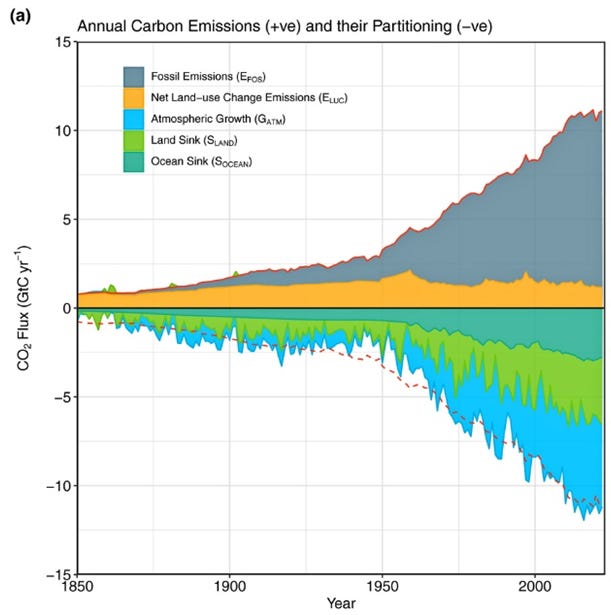
It is a known problem: there are “sinks” that absorb the CO₂ emitted by hydrocarbon burning so that only a fraction of it remains in the atmosphere (it is called the “airborne fraction.”) Remarkably, the sinks absorb more than half of the emissions, as you see in the figure below.
The “land sink” means carbon uptake by the land biosphere, whereas the “ocean sink” means carbon uptake in the form of both biomass and dissolved CO₂ in water (for those of you who are chemists, it becomes HCO3-). The difference is that the biomass sink generates oxygen while the seawater sink does not.
Land uptake could be the result of increased biomass (tree trunks, leaves. etc.) or be stored as soil carbon — mainly humus. From the figure, we see that it is about 2-3 Gt C/year. According to IPCC and the Carbon Project data, from 1950 the land sink adsorbed about 170 GtC. It is a huge amount compared to ~550 Gt C total Earth biomass (340 Gt C above-ground). The land biomass couldn’t possibly have increased so much. It was the increased metabolic activity of plants that stored carbon in the form of humus. In time, this humus will become inert kerogen and fossil fuels, and disappear from the ecosphere until pushed back into it by volcanoes or other geothermal activity. Note that increased photosynthesis activity also explains the mismatch between CO2 emissions and O2 decline.
It means that there exists a powerful feedback mechanism that makes the biosphere react to remove most of the CO₂ that humans are emitting into the atmosphere. It is a confirmation of the intuition of Gorshkov and Makarieva about the “biotic regulation” of the ecosphere.
It is a good thing that this feedback exists, but the problem is that all regulation mechanisms can be overloaded and overcome. The mechanism seems to have been working up until now, but as we continue emitting CO₂, there is evidence that it is stopping to work (see a recent article by John Rockstrom. At least, the buried carbon is unlikely to be re-emitted as CO₂ into the atmosphere, but if we keep emitting CO₂, the whole climate system risks being overwhelmed. Bad. But we already knew that.
____________________________________________________
How to calculate the oxygen concentration in ppm from the “per meg” values. Here is how the “per meg” thing is defined.
If we assume that the N2 concentration remains constant, we can calculate the “ppmv,” parts per million in volume as
With fo the reference oxygen concentration. There follows that a variation of 24 “per meg” corresponds to about 5 ppmv of oxygen.
_____________________________________________________________
Note: the first version of this paper contained an error in the conversion from Gt CO2 to GtC. Remarkably, Grok checked the paper but couldn’t detect the error. Instead, DeepSearch did identify the error and corrected it. Conclusion: if you want to check the data in a scientific paper, use deepsearch (Grok's cousin) but not Grok. h/t Duen Hsi-Yen







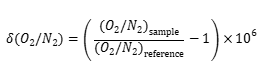

It remains somewhat mysterious where the CO2 goes, as in Anastassia Makarieva's last article.
Per Grok: "A critical error lies in the conversion factor: the post claims 1 ppm CO2 corresponds to 2.13 Gt of CO2, but standard sources, such as the Carbon Dioxide Information Analysis Center (Conversion Tables), state 1ppm CO2 equals 2.13 Gt of carbon. To find the CO2 mass, multiply by 44/12: 2.13 * (44/12) ≥ 7.82 Gt CO2 per ppm. This error affects calculations: the post suggests 36 Gt CO2 corresponds to 36 / 2.13 = 16.9ppmv per year (rounded to 17 pmv), but correctly, it's 36 / 7.82 ≥ 4.6 ppmv per year."
https://grok.com/share/bGVnYWN5_58103b9a-852b-49ab-ab73-3e6febf37299