What if we Burned Everything?
Are we suffocating? No, but we may still be doing something bad to the biosphere
Earth is an explosive mixture of burnable carbon and the oxygen needed to burn it. Could it actually burn completely in a single, gigantic outburst of flames? Hopefully not, but as Sir Thomas Browne never said, the limits of human evil, though a puzzling question, are not beyond all conjecture. In any case, Gaia knows best.
(note: this post was modified after publication to correct for a misinterpretation of the concept of “per Meg” concentration in the atmosphere. But its conclusion remain the same)
My interest in climate science started long ago, at a time when we were mostly worried about nuclear war. The theme of nuclear apocalypse was widely explored in the science fiction of the time, and I was very impressed by the novel "A Canticle for Leibowitz" by Walter M. Miller Jr., published in 1960. Describing the effects of a major nuclear war, Miller wrote:
"After that Earth had killed itself, it burned itself on a pyre. The flame mounted up and spumed over the sky. The clouds caught fire; the rain turned to ashes. And out of the ashes, a new Earth was born, and the old one was forgotten."
That sentence impressed my mind for a long time. Most of the discussion about climate rotates around the idea that we should burn less than what we are burning now. What would happen if we were to burn everything that can be burned?
Burning means combining atmospheric oxygen with carbon, and that necessarily requires drawing down oxygen from the atmosphere. It is happening; here are some data from the Mauna Loa Observatory, the same that provides data on CO2 concentrations (Source)
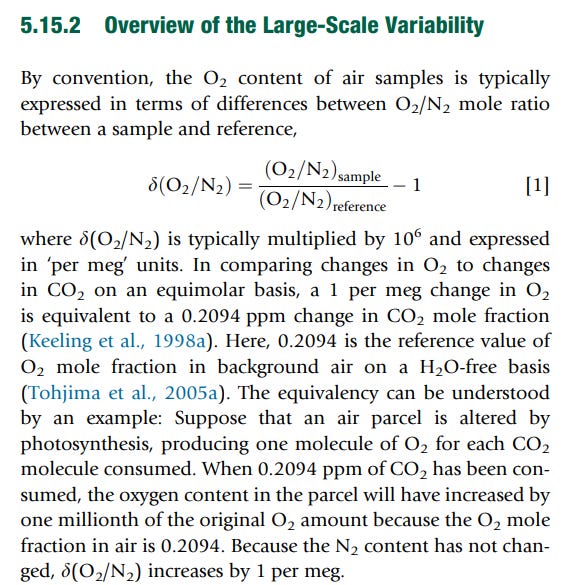
Here, oxygen is measured as 'per meg' which is said to be “δ(O2 /N2) multiplied by 10^6.” That is, the measurement is made as a ratio of the oxygen molar volume referred to a nitrogen reference to a standard that contains 0.2094% molar fraction of oxygen and the rest is nitrogen. Note also that since we are discussing of gases at the same pressure, the molar fraction and the volume fraction is the same. Taking this into account, the “per Meg” ratios can be compared to the ppmv values of CO2 by multiplyingby 0.2094 (See below (**)).
From the figure, we can say that during the past ten years or so (from 2012 to 2022), we lost roughly 250 “per meg” of oxygen, or 52.3 ppm. In other words, we are losing about 5.2 ppm of oxygen per year. Consider now that fossil fuels are generating about 6 ppm of carbon dioxide per year. The two numbers roughly match each other, so we can say that most of the decline in atmospheric oxygen concentration is due to fossil fuel burning.
Before you start worrying about that, think that the oxygen concentration in the atmosphere in weight is about 23% (21% in volume). That is, 230,000 parts per million. You see that a loss of 5 ppm is infinitesimal and you are not going to choke soon. There are many more and more pressing problems generated by the current CO2 increase in the atmosphere of about 2.7 ppmv per year.
But let’s go back to the initial question. How about burning the whole biosphere, trees, plants, animals, and everything? There are roughly 550-600 billion metric tons (Gt, or petagrams, Pg) of carbon in terrestrial biomass. Considering that carbon has an atomic weight of 12, that means 50 Petamoles (50*10^15 gram-molecules). One carbon atom reacts with one molecule of oxygen (O2) to form CO2. Now, consider that Oxygen has a molecular weight of 32, So, 50 Petamoles of oxygen correspond to 1600 Pg, or 1600 Gtons. The total mass of the oxygen in the atmosphere is much larger; it is 1.2×10^6 Pg.
It means that burning the whole biosphere would mean losing a little more than 0.1% of the oxygen mass of the atmosphere. No worry that anyone would suffocate.
That generates a question: where does the other 99.9% of the oxygen come from? There is no other significant oxygen source in the atmosphere than photosynthesis, and for each Oxygen molecule formed, there must have been a CO2 molecule transformed into an organic molecule. Where has all this carbon gone?
It is still there, but it is not in the biosphere. It exists in various solid forms or gaseous/liquid ones trapped in Earth’s crust. They are the fossil fuels (coal, oil, and gas) and methane hydrates. But, by far, the most abundant form is “kerogen” solid, waxy hydrocarbons usually embedded in shale rocks. Their mass is estimated as approximately 10^22 Pg of carbon, which nicely matches the mass of oxygen, although slightly larger. It was generated in billions of years of photosynthetic activity of the biosphere.
If we were to burn all the kerogen in the crust, then we would reset the timer to zero and really consume all the oxygen in the atmosphere. Would you think people could be so stupid to do something like that? Well, never underestimate human idiocy when it is mixed with human evil. Fortunately, kerogen burns poorly and must be extracted from the shale rock that contains it. It is a complicated and expensive process, but it is done in some places; it is a process called retorting*. So, you see, Walter Miller’s description of how the human species destroyed themselves by burning everything was not completely outside the range of the possibilities of human evil.
There is much more than this in the story of how oxygen and carbon balance each other in the atmosphere, but we’ll see that in the next post.
(*) note that oil from shales is not the same thing as “shale oil,” which should be more correctly defined as “tight oil.” But the same name is used for both, and that creates a lot of confusion. In any case, they are both evil things.
(**). Here is how the “per Meg” unit is described. It is tricky, and in order to understand it you have to access a paper that’s behind a paywall. Anyway, here is the definition.







As I knew, "Oxygen on Earth primarily came about through the process of photosynthesis performed by ancient bacteria called cyanobacteria, which released oxygen as a byproduct while using sunlight to convert carbon dioxide and water into energy, marking a significant event known as the "Great Oxidation Event" where oxygen levels in the atmosphere began to rise significantly; this process occurred roughly 2.4 to 2.3 billion years ago."
That's how Oxygen concentration rose from 1% to 21 % (was even higher).
That's a huge, huge amount! And may not even require regular “replenishment” and production of “new oxygen”.
And in the present days - The waters (oceans) of the world are the main oxygen generators of the biosphere; their algae are estimated to replace about 90 percent of all oxygen used.
Again- as I know- plants produce O2 in a day (light) time (photosynthesis)... And in the night time they "consume" >50% of released O2)))) And they emit CO2 in the night)) They "breath"! (That is why "Nature" emits >200Gt of C per year (750Gt CO2) - and sequester the same amount!!
So, it's complicated:::))))